Overview
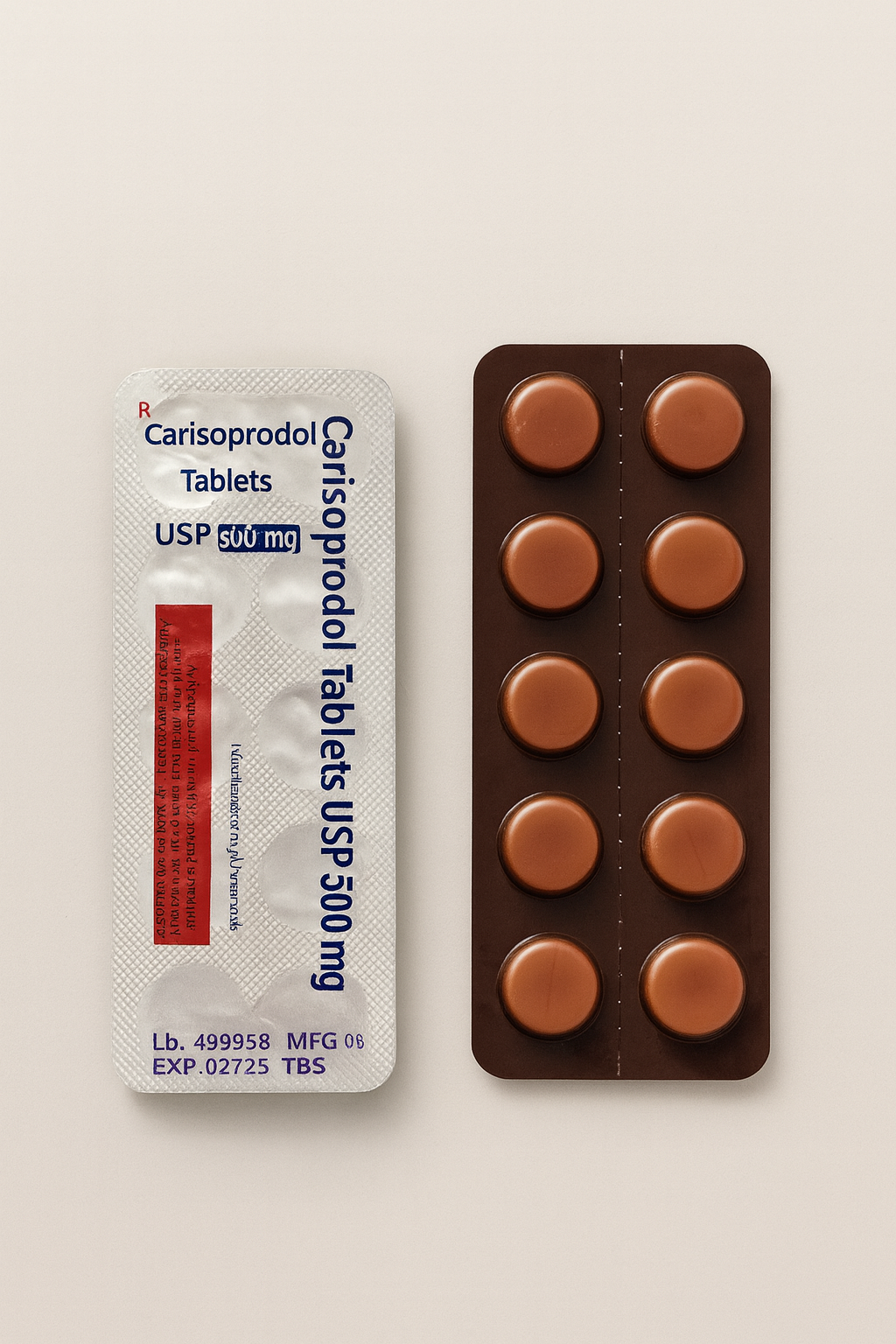
Pain-O-Soma contains Carisoprodol 500 mg, a centrally acting skeletal-muscle relaxant used for the relief of acute, painful musculoskeletal conditions.
It functions by interrupting interneuronal communication within the spinal cord and descending reticular formation, resulting in reduced skeletal-muscle tone and relief from discomfort associated with muscle spasm.
The product is manufactured under Good Manufacturing Practice (GMP) standards by HAB Pharmaceuticals & Research Ltd., India. Each uncoated tablet delivers rapid onset of muscle relaxation and pain relief, with a clinical duration of action of roughly 4 to 6 hours.
Pain-O-Soma is intended for short-term therapy—as an adjunct to rest, physical therapy, and other supportive measures—in the treatment of acute musculoskeletal disorders.
Pharmacological Classification
| Parameter | Details |
|---|---|
| Generic Name | Carisoprodol |
| Brand Name | Pain-O-Soma |
| Strength | 500 mg per tablet |
| Therapeutic Class | Skeletal-muscle relaxant / Analgesic adjunct |
| Pharmacologic Class | Centrally acting carbamate derivative |
| Dosage Form | Oral tablet |
| Route of Administration | Oral |
| Manufacturer | HAB Pharmaceuticals & Research Ltd. |
| Regulatory Category | Prescription-only medicine (Rx) |
| Pharmacopoeial Standard | USP / I.P. compliant |
Mechanism of Action
Carisoprodol’s exact molecular mechanism is not fully elucidated, but experimental evidence supports a central nervous system depressant effect at both spinal and supraspinal levels.
-
The drug modifies interneuronal activity in the reticular formation and spinal cord, reducing polysynaptic reflexes responsible for muscle tone.
-
Its primary metabolite, meprobamate, exhibits anxiolytic and sedative properties that may contribute to its overall muscle-relaxant effect.
-
The resulting action decreases skeletal-muscle spasm, alleviates pain perception, and improves mobility.
Key pharmacodynamic effects:
-
Sedation and skeletal-muscle relaxation.
-
Reduction of reflex arc excitability.
-
No direct effect on the neuromuscular junction or skeletal muscle fibers.
Pharmacokinetic Profile
| Parameter | Description |
|---|---|
| Absorption | Rapidly absorbed from the gastrointestinal tract; peak plasma levels within 1 – 1.5 hours. |
| Bioavailability | ~80 %. |
| Protein Binding | ~60 %. |
| Metabolism | Hepatic, primarily by CYP2C19, to meprobamate and other minor metabolites. |
| Elimination Half-life | Carisoprodol ≈ 2 hours; meprobamate ≈ 10 hours. |
| Excretion | Renal (metabolites and small amounts unchanged). |
| Onset of Action | 30 minutes after ingestion. |
| Duration of Effect | 4 – 6 hours. |
Clinical Pharmacology
Steady-state is achieved within 2 days of regular dosing. The parent compound’s rapid clearance minimizes accumulation, though the active metabolite meprobamate may persist longer, necessitating caution with repeated high-dose or prolonged therapy.
Therapeutic Indications
Pain-O-Soma (Carisoprodol 500 mg) is indicated for:
-
Relief of acute, painful musculoskeletal conditions such as:
-
Muscle strain or sprain
-
Low-back pain
-
Neck and shoulder spasm
-
Myofascial pain syndrome
-
Post-traumatic muscle spasm
-
-
Adjunctive therapy to:
-
Rest and immobilization of the affected part.
-
Physical therapy or rehabilitation.
-
Not intended for long-term use or chronic musculoskeletal disorders.
Target Audience
-
Adult patients (≥ 18 years) experiencing acute muscle spasm or injury-related pain.
-
Healthcare professionals—orthopedic physicians, general practitioners, physiatrists—requiring short-acting muscle relaxant therapy.
-
Post-injury and rehabilitation programs requiring pharmacologic support to facilitate therapy compliance and pain-free movement.
Dosage and Administration
Always individualize dosing based on patient condition, age, and tolerability.
| Indication | Recommended Dose | Frequency | Maximum Duration |
|---|---|---|---|
| Acute musculoskeletal pain | 250 – 350 mg (standard) or 500 mg tablet | Three times daily and at bedtime | ≤ 2 – 3 weeks |
Administration Guidelines
-
Administer orally with or without food.
-
Night-time dose aids sleep and muscle relaxation.
-
If dose missed, take as soon as remembered unless near next scheduled dose.
-
Avoid abrupt discontinuation after prolonged therapy to minimize withdrawal symptoms.
Problem Addressed
Acute musculoskeletal pain arises from overexertion, trauma, or strain, leading to muscle spasm, inflammation, and impaired mobility.
Such conditions involve increased muscle-fiber excitability and reflex activity within the spinal cord, producing a pain–spasm–pain cycle that delays recovery.
Pain-O-Soma interrupts this cycle by:
-
Depressing spinal reflexes, reducing muscle tone.
-
Alleviating central perception of pain.
-
Facilitating rest and rehabilitation.
Its combined analgesic and sedative actions make it a clinically useful short-term adjunct for restoring normal function after musculoskeletal injury.
Key Clinical Benefits
-
Rapid relief from acute muscle spasm and associated pain.
-
Improved range of motion and flexibility.
-
Facilitates physiotherapy by reducing discomfort during exercises.
-
Short onset and moderate duration allow individualized daytime or bedtime dosing.
-
Predictable pharmacokinetics for safe short-term use.
-
Favorable tolerability profile when administered within recommended duration.
Contraindications
-
Hypersensitivity to Carisoprodol or meprobamate.
-
Acute intermittent porphyria.
-
Severe hepatic or renal impairment.
-
History of drug abuse, addiction, or dependence.
-
Concomitant use of other sedative–hypnotic drugs without medical supervision.
Warnings and Precautions
-
Duration of therapy:
Limit to 2 – 3 weeks; efficacy beyond this period not established. -
CNS Depression:
Causes drowsiness, dizziness; caution with driving or operating machinery. -
Abuse and Dependence:
Risk increases with prolonged use or high doses. Use only under medical supervision. -
Withdrawal Symptoms:
May include anxiety, tremor, or insomnia after abrupt cessation; taper gradually. -
Elderly Patients:
Greater risk of sedation and falls; initiate at lower dose if therapy essential. -
Hepatic / Renal Impairment:
Accumulation possible; use with caution. -
Pregnancy and Lactation:
Safety not established; use only if potential benefit justifies risk. -
Pediatric Use:
Not recommended for individuals under 18 years.
Adverse Reactions
| System Affected | Common (>1 %) | Uncommon / Rare |
|---|---|---|
| Central Nervous System | Drowsiness, dizziness, headache | Confusion, ataxia, tremor |
| Gastrointestinal | Nausea, vomiting | Abdominal discomfort |
| Allergic / Dermatologic | Pruritus, erythema | Urticaria, rash |
| Cardiovascular | Hypotension (mild, transient) | Tachycardia (rare) |
| Others | Somnolence, blurred vision | Dependence with long use |
Most effects are mild and transient. Discontinue therapy if severe CNS depression, rash, or idiosyncratic reaction occurs.
Drug Interactions
| Drug Class | Interaction Type | Clinical Outcome / Recommendation |
|---|---|---|
| CNS depressants (opioids, benzodiazepines, alcohol) | Pharmacodynamic synergy | Additive sedation and respiratory depression — avoid or monitor closely |
| Antiepileptics / Barbiturates | Pharmacodynamic synergy | Enhanced CNS depression |
| CYP2C19 Inhibitors (omeprazole, fluvoxamine) | Pharmacokinetic effect | Increased Carisoprodol exposure |
| CYP2C19 Inducers (rifampicin, carbamazepine) | Pharmacokinetic effect | Reduced plasma concentration and efficacy |
| Other muscle relaxants | Pharmacodynamic | Avoid duplication; risk of oversedation |
Overdose Management
Symptoms: Severe sedation, confusion, ataxia, respiratory depression, hypotension, or coma (rare).
Management:
-
Supportive care—airway protection, monitoring of vitals.
-
Gastric lavage or activated charcoal if early ingestion.
-
Intravenous fluids for hypotension.
-
Hemodialysis not routinely effective but may help remove meprobamate metabolite.
Special Populations
| Population | Considerations |
|---|---|
| Elderly | Use lower initial doses; higher risk of sedation. |
| Renal Impairment | Dose adjustment or alternative therapy recommended. |
| Hepatic Impairment | Increased exposure due to reduced metabolism; use cautiously. |
| Pregnancy / Lactation | Insufficient data; avoid unless necessary. |
| Pediatrics | Not established; avoid. |
Clinical Efficacy Summary
Clinical studies demonstrate that Carisoprodol 500 mg, administered three to four times daily for ≤ 2 weeks, provides statistically significant improvements in:
-
Muscle-spasm reduction (measured by EMG and clinical scales).
-
Pain scores compared with placebo or NSAID monotherapy.
-
Functional recovery time post-injury.
-
Sleep quality due to sedative–muscle-relaxant synergy.
Combination with non-pharmacologic measures (rest, physiotherapy) yields superior outcomes versus medication alone.
Carisoprodol has not demonstrated benefit in chronic pain or spasticity of neurologic origin.
Storage and Handling
-
Store at 25 °C (77 °F); excursions 15–30 °C permitted.
-
Protect from moisture and direct light.
-
Keep in original blister pack until use.
-
Shelf life: 24 months from manufacturing date.
-
Keep out of reach of children.
Patient Counselling Information
Healthcare professionals should instruct patients to:
-
Take medication only as prescribed and for the shortest duration necessary.
-
Avoid alcohol or sedative co-administration.
-
Expect possible drowsiness—do not drive or operate machinery.
-
Combine pharmacologic therapy with rest, stretching, and physical therapy.
-
Report any allergic reactions, confusion, or excessive sedation immediately.
-
Do not share medication or self-adjust the dose.
-
Discontinue gradually if used longer than 2 weeks.
Conclusion
Pain-O-Soma (Carisoprodol Tablets USP 500 mg) is a centrally acting skeletal-muscle relaxant providing short-term relief from acute musculoskeletal pain and spasm.
Its mechanism—modulation of interneuronal transmission in the spinal cord—offers effective reduction of muscle tone and pain perception.
When prescribed responsibly and combined with rehabilitative therapy, Pain-O-Soma delivers a clinically validated solution for acute musculoskeletal disorders while maintaining a strong safety and tolerability record under medical supervision.
Reviews
There are no reviews yet.