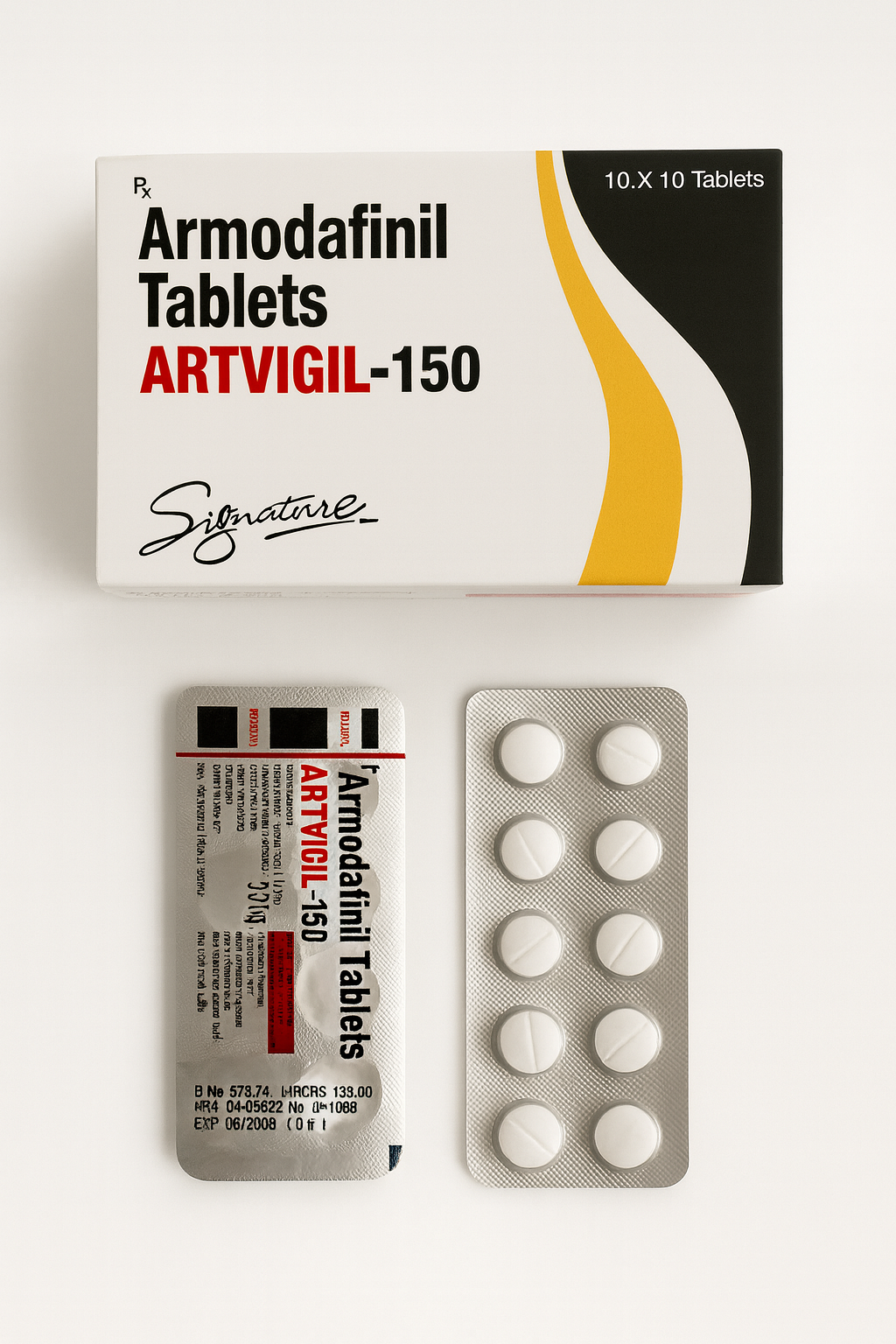
ARTVIGIL-150 (Armodafinil Tablets 150 mg) – Medical Information and Safe-Use Overview
Overview
ARTVIGIL-150 contains Armodafinil 150 mg, a prescription medicine developed to promote wakefulness in adults experiencing excessive daytime sleepiness (EDS) associated with medical sleep-wake disorders.
It belongs to the pharmacologic class known as wakefulness-promoting agents (eugeroics).
Armodafinil is the R-enantiomer of modafinil, offering a longer half-life and smoother pharmacodynamic profile.
It is prescribed to improve alertness in patients diagnosed with narcolepsy, obstructive sleep apnea (OSA), or shift-work sleep disorder (SWSD) under professional supervision.
Pharmacological Classification
| Category | Description |
| Therapeutic class | Central nervous-system stimulant / Eugeroic |
| Pharmacological class | Wakefulness-promoting agent |
| Active ingredient | Armodafinil 150 mg |
| Dosage form | Oral tablet |
| Manufacturer / Labeler | Signature Pharmaceuticals |
| Regulatory status | Prescription-only (Schedule H or equivalent) |
| Packaging | 10 × 10 tablet blisters |
Mechanism of Action
Although the precise mechanism is not fully understood, Armodafinil is believed to enhance wakefulness by modulating multiple neurotransmitter systems, including:
- Dopaminergic activity: Inhibits dopamine reuptake via the dopamine transporter (DAT), increasing extracellular dopamine levels in the brain’s wake-promoting regions.
- Histaminergic and orexin pathways: Stimulates hypothalamic neurons responsible for arousal and alertness.
- Glutamate and GABA balance: Enhances excitatory glutamate transmission while decreasing inhibitory GABA output, supporting cortical activation.
Unlike amphetamine stimulants, Armodafinil lacks significant effects on peripheral sympathomimetic pathways, producing wakefulness without pronounced jitteriness or rebound fatigue.
Clinical Uses and Indications
ARTVIGIL-150 (Armodafinil 150 mg) is indicated for the management of:
- Narcolepsy – A chronic neurological disorder characterized by uncontrollable sleep episodes, cataplexy, and excessive daytime drowsiness.
- Obstructive Sleep Apnea (OSA) – Used as adjunctive therapy to continuous positive airway pressure (CPAP) to combat residual sleepiness.
- Shift-Work Sleep Disorder (SWSD) – For individuals whose work schedules disrupt normal circadian rhythms, causing sleepiness during waking hours.
Note: Armodafinil treats sleepiness but does not cure the underlying disorder or replace adequate sleep.
Pharmacokinetic Profile
| Parameter | Description |
| Absorption | Rapid; peak plasma concentration in ~2 hours (fasted). |
| Bioavailability | Comparable to modafinil (~90%). |
| Protein binding | 60 %. |
| Metabolism | Hepatic; primarily via CYP3A4, minor roles of CYP2C19 and CYP2C9. |
| Half-life | 12–15 hours (longer than modafinil). |
| Excretion | Mainly renal (metabolites). |
Dosage and Administration
Dosage must be individualized under supervision of a physician familiar with sleep-wake disorders.
Adults (≥ 17 years):
| Indication | Typical Dosage | Timing |
| Narcolepsy / OSA | 150 mg once daily | In the morning |
| Shift-Work Sleep Disorder | 150 mg once daily | About 1 hour before work shift |
Administration Notes:
- Tablets should be swallowed whole with water.
- May be taken with or without food (food slightly delays absorption).
- Avoid evening dosing to prevent insomnia.
- Do not exceed prescribed dose.
Discontinuation should be discussed with the clinician; abrupt cessation rarely causes withdrawal but can re-induce sleepiness.
Target Population
- Adults clinically diagnosed with EDS due to narcolepsy, OSA, or SWSD.
- Patients requiring improved daytime alertness and performance despite underlying sleep pathology.
- Under physician supervision; not intended for healthy individuals seeking performance enhancement.
Therapeutic Benefits (When Used Correctly)
- Promotes Wakefulness: Reduces unplanned sleep episodes, maintaining alertness during required wake periods.
- Improves Cognitive Performance: Enhances concentration, attention span, and executive functioning in affected patients.
- Supports Productivity: Enables better occupational and social functioning despite sleep-wake disorders.
- Adjunct to CPAP in OSA: Counteracts residual fatigue when airway therapy alone is insufficient.
- Non-Amphetamine Profile: Provides stimulation with minimal euphoric or rebound effects when compared to traditional stimulants.
Contraindications
- Known hypersensitivity to armodafinil, modafinil, or excipients.
- History of allergic rash or multiorgan hypersensitivity to modafinil/armodafinil.
- Uncontrolled hypertension or cardiac arrhythmia (use with caution).
- Pregnancy and lactation (safety not fully established).
Warnings and Precautions
- Dermatologic Reactions
Serious rashes (including Stevens–Johnson syndrome) have been reported rarely. Discontinue at the first sign of rash.
- Psychiatric Symptoms
Monitor for anxiety, agitation, mania, or hallucinations, especially in patients with prior mental-health conditions.
- Cardiovascular Caution
Use carefully in patients with structural heart disease, recent myocardial infarction, or uncontrolled hypertension.
- Sleep Hygiene
Medication should complement, not replace, sufficient rest and adherence to physician-directed sleep-therapy regimens.
- Substance Use Potential
Armodafinil shows low but measurable potential for misuse; prescribers should assess risk factors before initiating therapy.
- Contraceptive Interaction
Induces CYP3A4, which may reduce effectiveness of hormonal contraceptives. Alternative methods recommended.
- Driving and Machinery
May affect concentration in some individuals until response is known; caution advised.
Adverse Reactions
Common (≥ 5 %):
- Headache
- Nausea
- Insomnia
- Dizziness
- Dry mouth
Less Common:
- Anxiety, irritability
- Palpitations, elevated BP
- Gastrointestinal upset
Rare but Serious:
- Rash, allergic reaction
- Psychiatric disturbances (mania, hallucination)
- Severe fatigue on withdrawal
Adverse effects are typically dose-related and often resolve with continued use or dosage adjustment.
Drug Interactions
| Drug Category | Effect / Guidance |
| CYP3A4 substrates (e.g., oral contraceptives, cyclosporine) | Reduced effectiveness; use alternative therapy. |
| CYP3A4 inhibitors (ketoconazole, erythromycin) | May increase armodafinil exposure. |
| CYP2C19 substrates (diazepam, phenytoin) | Elevated plasma levels; monitor. |
| CNS stimulants or caffeine | Additive stimulation possible. |
| Antiepileptics (carbamazepine) | May lower armodafinil concentration. |
Overdose Management
Symptoms: Restlessness, insomnia, agitation, tachycardia, or nausea.
Treatment: Supportive care; gastric lavage if early ingestion; cardiovascular monitoring. No specific antidote.
Use in Specific Populations
| Population | Guidance |
| Pregnancy | Limited human data; use only if benefits outweigh risks. |
| Lactation | Excretion in milk unknown; avoid or monitor infant. |
| Pediatrics (< 17 years) | Safety and efficacy not established. |
| Geriatrics (> 65 years) | Use lower initial doses; slower clearance observed. |
| Hepatic impairment | Dose reduction (half-strength) recommended. |
| Renal impairment | Mild effects; adjust only if severe. |
Storage and Handling
- Store at 20–25 °C (68–77 °F), away from moisture and direct sunlight.
- Keep in original blister until use.
- Keep out of reach of children.
- Dispose of expired or unused tablets via approved pharmaceutical-waste channels.
Patient Education and Counseling Points
Healthcare providers should advise patients to:
- Take the medicine exactly as prescribed—never double the dose.
- Avoid driving until individual response is known.
- Maintain regular sleep patterns and good sleep hygiene.
- Inform the doctor about all current medications, supplements, and contraceptive methods.
- Report new rash, chest pain, or mood changes immediately.
- Do not share medication with others, even if they have similar symptoms.
Problem Addressed
Excessive daytime sleepiness (EDS) is a hallmark symptom of narcolepsy, sleep apnea, and shift-work disorder.
It leads to poor concentration, cognitive lapses, accidents, and impaired productivity.
Traditional stimulants can cause jitteriness, cardiovascular strain, and dependence.
Armodafinil 150 mg provides a modern pharmacological approach by targeting wake-promoting pathways selectively, allowing patients to remain alert and focused without excessive stimulation.
When integrated into a comprehensive sleep-disorder management plan, it improves alertness, occupational performance, and overall quality of life.
Desired Outcomes of Therapy
- Restoration of normal wakefulness during daytime or work hours.
- Reduced fatigue and microsleep episodes.
- Improved cognitive clarity and productivity.
- Safer occupational functioning for individuals in safety-sensitive roles.
- Better psychosocial well-being through improved alertness.
Reviews
There are no reviews yet.