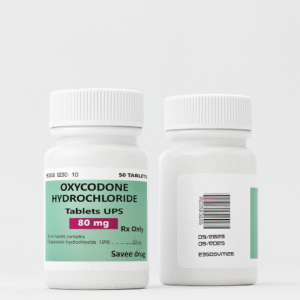
Oxycodone Hydrochloride Tablets USP 80 mg – Clinical Overview and Safe-Use Information
General Information
Oxycodone Hydrochloride Tablets USP 80 mg are an oral, prescription-only opioid analgesic formulated for the management of moderate to severe pain requiring continuous, around-the-clock opioid therapy.
This medication contains Oxycodone Hydrochloride, a semisynthetic opioid agonist derived from thebaine and classified as a Schedule II controlled substance in many countries.
It acts primarily on the central nervous system (CNS) to relieve pain by binding to μ-opioid receptors in the brain and spinal cord.
Each tablet delivers a controlled 80 mg dose intended for patients who are opioid-tolerant and require sustained analgesia under strict medical supervision.
Active Ingredient and Composition
| Component | Specification |
| Active ingredient | Oxycodone Hydrochloride 80 mg |
| Inactive ingredients | May include lactose monohydrate, microcrystalline cellulose, magnesium stearate, colloidal silicon dioxide, and other excipients per manufacturer formulation |
| Dosage form | Oral tablet |
| Manufacturer / Labeler | Savee Drug (pharmaceutical division complying with USP standards) |
| Pack size | 50 tablets per container |
| Strength | 80 mg per tablet |
Mechanism of Action
Oxycodone is a full opioid agonist with primary activity at the μ-opioid receptor.
Binding to these receptors alters the perception of and emotional response to pain by inhibiting ascending pain pathways within the CNS.
It also causes generalized CNS depression, producing analgesia, sedation, and a sense of calm, while at higher doses it may depress respiration.
In clinical settings, Oxycodone provides strong analgesia comparable to morphine but with slightly greater oral bioavailability.
Indications and Clinical Use
Oxycodone Hydrochloride Tablets USP 80 mg are indicated for:
- Management of severe chronic pain that cannot be controlled by non-opioid or combination analgesics.
- Post-operative pain following major surgery where extended analgesic coverage is required.
- Cancer-related pain and palliative-care settings demanding continuous opioid therapy.
- Trauma or orthopedic pain unrelieved by weaker medications.
Important:
The 80 mg strength is recommended only for opioid-tolerant patients—those who have been receiving and tolerating daily opioid doses equivalent to at least 60 mg of oral morphine for a minimum of one week.
Clinical Benefits When Used Appropriately
- Provides consistent, long-lasting pain relief over several hours.
- Improves comfort and functional ability in patients with severe, persistent pain.
- Facilitates better sleep and quality of life by minimizing breakthrough pain episodes.
- Enables continuity of care in oncology, hospice, and chronic-pain management programs.
Dosage and Administration
Caution: Dosing must be individualized and initiated under close clinical supervision by a qualified physician experienced in opioid therapy.
- Adults (opioid-tolerant): Typical initial doses vary based on prior opioid exposure. Conversion from other opioids should use an equianalgesic chart.
- Route: Oral; swallow tablets whole with water.
- Do not crush, chew, or dissolve tablets—doing so can cause rapid release and absorption of a potentially fatal dose.
- Frequency: Usually every 12 hours, but may differ depending on patient response and formulation type.
- Titration: Adjust gradually to achieve adequate analgesia with minimal side effects.
- Discontinuation: Taper slowly to avoid withdrawal symptoms.
Warnings and Precautions
- Addiction, Abuse, and Misuse
Oxycodone carries a high potential for abuse and psychological dependence. Prescribe only when benefits outweigh risks and monitor all patients regularly. - Life-Threatening Respiratory Depression
Serious, possibly fatal respiratory depression may occur, especially during initiation or dose escalation. Administer the lowest effective dose. - Accidental Ingestion
Even one dose of the 80 mg tablet can be fatal if taken by a child or opioid-naïve adult. - Neonatal Opioid Withdrawal Syndrome
Prolonged use during pregnancy may cause withdrawal in the newborn. Use only if alternatives are inadequate. - Interactions with CNS Depressants
Alcohol, benzodiazepines, sedatives, or other opioids may enhance CNS depression and respiratory risk. - Elderly and Debilitated Patients
Use with caution; sensitivity to respiratory and CNS effects may be increased. - Driving and Machinery Operation
May impair mental and physical abilities. Patients should refrain from operating vehicles or heavy machinery until effects are known. - Dependence and Withdrawal
Abrupt cessation after prolonged use may cause withdrawal symptoms—restlessness, rhinorrhea, sweating, and abdominal cramps. Gradual tapering is mandatory.
Adverse Reactions
Common Side Effects
- Drowsiness, dizziness
- Nausea or vomiting
- Constipation
- Dry mouth
- Headache
- Sweating
Serious Adverse Effects
- Respiratory depression
- Severe hypotension
- Confusion or hallucinations
- Bradycardia
- Seizures (rare)
Adverse reactions are generally dose-related and may be minimized by careful titration and concurrent management (e.g., stool softeners for constipation).
Drug Interactions
- CYP3A4 inhibitors (e.g., clarithromycin, ketoconazole) → may increase plasma oxycodone concentration and toxicity risk.
- CYP3A4 inducers (e.g., rifampin, carbamazepine) → may reduce efficacy.
- Benzodiazepines or alcohol → enhanced CNS and respiratory depression.
- Monoamine-oxidase inhibitors (MAOIs) → potential serotonin syndrome or opioid toxicity.
- Muscle relaxants and antipsychotics → additive sedation.
Always review all concomitant medications before initiating therapy.
Overdose Management
Symptoms:
- Extreme somnolence
- Pinpoint pupils
- Slow or shallow breathing
- Cyanosis
- Loss of consciousness
Treatment:
- Immediate medical attention is critical.
- Support airway and ventilation.
- Administer naloxone (opioid antagonist) as directed.
- Monitor continuously for recurrence due to oxycodone’s longer half-life compared with naloxone.
Use in Specific Populations
| Population | Recommendation |
| Pregnancy | Avoid unless absolutely necessary; risk of neonatal withdrawal. |
| Lactation | Passes into breast milk; may cause respiratory depression in infants. |
| Pediatrics | Not established; 80 mg strength contraindicated. |
| Geriatrics | Start with lower doses; monitor for sedation and confusion. |
| Renal/Hepatic impairment | Reduce initial dosage; monitor closely. |
Storage and Handling
- Store at 25 °C (77 °F); excursions permitted 15–30 °C (59–86 °F).
- Keep tightly closed and protected from moisture and light.
- Controlled-substance storage: Maintain in a locked, secure cabinet per local narcotic-control regulations.
- Dispose of unused tablets through authorized drug take-back programs; never flush down toilets.
Conclusion
Oxycodone Hydrochloride Tablets USP 80 mg remain an essential option in modern pain management when used judiciously.
They deliver potent, sustained analgesia for severe pain states unresponsive to other therapies, provided they are prescribed and monitored within a comprehensive, multidisciplinary care plan.
For healthcare providers and patients alike, understanding the benefits, risks, and safe-use protocols of Oxycodone is crucial to achieving effective pain relief while minimizing potential harm.

Reviews
There are no reviews yet.